Objective:
To evaluate whether an online engagement platform for chronic hives / urticaria (CU) accessible to diverse patient populations and focused on symptom tracking, assessment of psychiatric comorbidities, and clinician feedback improves immediate and long-term CU symptom control.
Background and Problem
Urticaria or hives is a common affliction that affects 25% of the general population. A subset of patients with urticaria develop chronic urticaria (CU), which has an estimated prevalence of 0.5-5% and incidence of 1.4% per year in the general population and is characterized by recurrent pruritic hives and/or painful angioedema for six weeks or more. The biology of CU is not well-understood, although a subset of patients may have an autoimmune basis to their CU.
CU has a significant detrimental impact on quality of life as CU disrupts patients’ activities of daily living, work, and sleep. Indeed, one-third to one-half of CU patients experience depression and anxiety, and the health status of CU patients is comparable to coronary artery disease patients awaiting bypass surgery. Many patients do not receive adequate psychosocial care.
Achieving symptom control during the first year after CU onset is recommended, as data suggest that early symptom control is associated with improved long-term symptom control. Unfortunately, despite the fact that there are effective CU therapies available, achieving early symptom control remains one of the biggest challenges in CU.
Cultural and linguistic barriers prevent patients from receiving the frequent clinician feedback needed for symptom assessment and treatment. Another significant barrier to achieving early symptom control is inadequate access to allergists. Patients report 3-6 month wait times to be seen at allergy clinics (UCSF, other academic centers, and private practices). This wait period encroaches on that early time period when achieving symptom control is critical. After patients have been seen by an allergist, distance can prevent patients from receiving the frequent follow-up needed for symptom assessment and treatment.
Proposed Solution
An online engagement platform with symptom tracking and clinician feedback would address the major barriers to adequate care – distance, cultural and linguistic barriers, and insufficient access to allergists. It would allow allergists to manage a diverse population of patients remotely and enable early symptom control critical for long-term treatment success. Existing apps created through the American College of Dermatology, Genentech/Novartis, and eResearchTechnology are only available in English and are not adequate for meaningful patient follow-up.
One emphasis will be on creating a platform with a culturally and linguistically appropriate design such that non-English-speaking patients from diverse cultural backgrounds can be included in the study cohort. We will start by conceptualizing the initial platform design with existing symptom and quality-of-life measurement tools that have been translated into Chinese and Spanish and have been validated in Chinese and Latin@ populations. From there, we plan to use semi-structured interviews of chronic hive patients in our clinics (initially English-speaking and Chinese-speaking) to guide platform design so that it is tailored to cultural and linguistic needs. If our initial pilot studies are successful, our plan is to expand the platform to include culturally and linguistically appropriate tools for Spanish-speaking Latin@ patients. In this way, we hope to potentially adapt existing symptom and quality-of-life measurement tools to culturally diverse populations.
Our proposed platform and studies would assess the cultural appropriateness of smartphone-based health data and best strategies for patient engagement across diverse populations.
A CU cohort lends itself well to this type of platform.
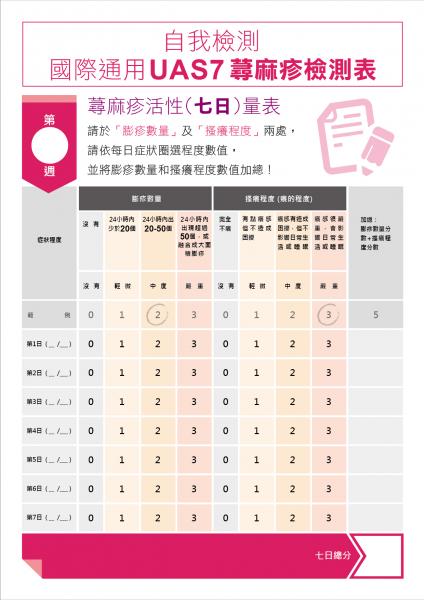
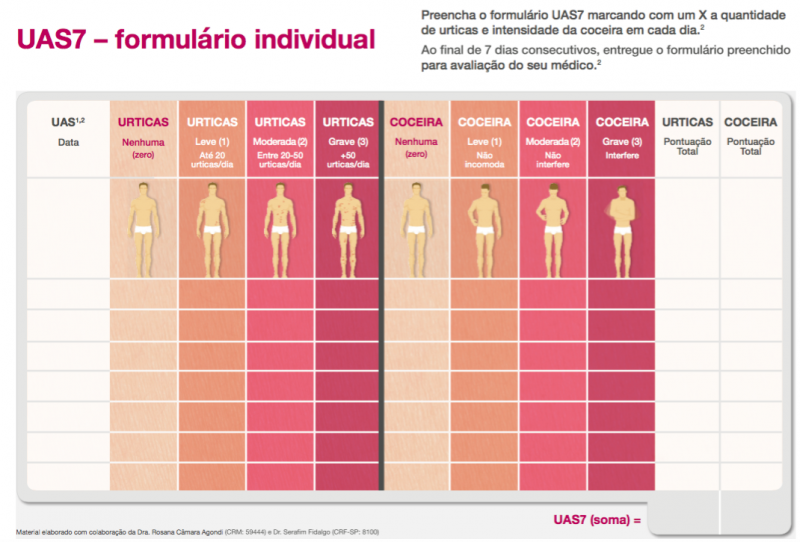
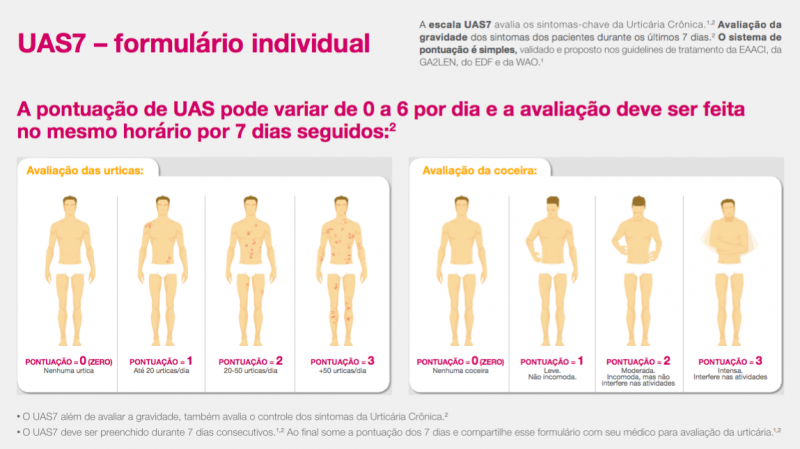
- There are well-validated tools to measure symptoms. The Urticaria Activity Score (UAS-7) and Itch Severity Scale (ISS) are recommended for use in routine practice and in clinical trials to measure disease severity. Symptom tracking allows patients to better identify non-allergic triggers and specific physical triggers, which affect 25% of patients with CU.
- There are validated quality of life tools such as the Chronic Urticaria Quality of Life Questionnaire (CU-Q2OL) and Dermatology Life Quality Index (DLQI).
- CU therapies are known to be effective in up to 97% of patients, and the main difficulty with symptom control is access to care and adequate follow-up. Therefore, an online platform that enables early access and frequent follow-up is primed for success.
Validated CU tools have been translated into multiple languages and studied in diverse populations, which supports the creation of a platform with a culturally and linguistically appropriate design and function.
There are tools that have been translated into Chinese and studied in Chinese populations. There are also tools that we would need to translate and do field testing in our population to make sure they are understandable and reliable. We have outlined these tools below. We would ask for community partner input (patients, allergists, primary care physicians) to determine which tools would be preferred for use in the app. Lulu Tsao, who is fluent in English and Chinese, will conduct semi-structured interviews with Chinese and Chinese-American patients to gauge their interest in an app, how they would want to use an app (symptoms, messaging, photos), and whether they would want recommendations from an allergist via an app.
- The UAS has been used in research studies in both mainland China and Taiwan. It was used in a cross-sectional survey of about 3000 patients in tertiary hospitals in different provinces.
- The DLQI has also been used for Chinese patients and an official Chinese (traditional and simplified) version is available online. This version was studied among 148 patients with CU in Taiwan and found to have high reliability.
- The Urticaria Control Test (UCT) is available in 30 languages and consists of only 4 questions, although the translated versions are not readily available.
- The CU-Q20L has not been translated or validated in Chinese populations, and translation and field testing with our population is needed to make sure it is understandable and reliable.
If an initial pilot in a Chinese-speaking population is successful, our plan is to also expand the platform to include Spanish-speaking patients. At that point, similar review of existing symptom and quality of life scoring tools and discussions with the appropriate community partners will be undertaken to determine which tools are most suitable for this patient population. In terms of already validated tools, the UAS and UAS7 were validated by researchers in Spain in a multicenter study of 166 patients and found to have good internal consistency, reliability, and sensitivity to change.
Chinese UAS7
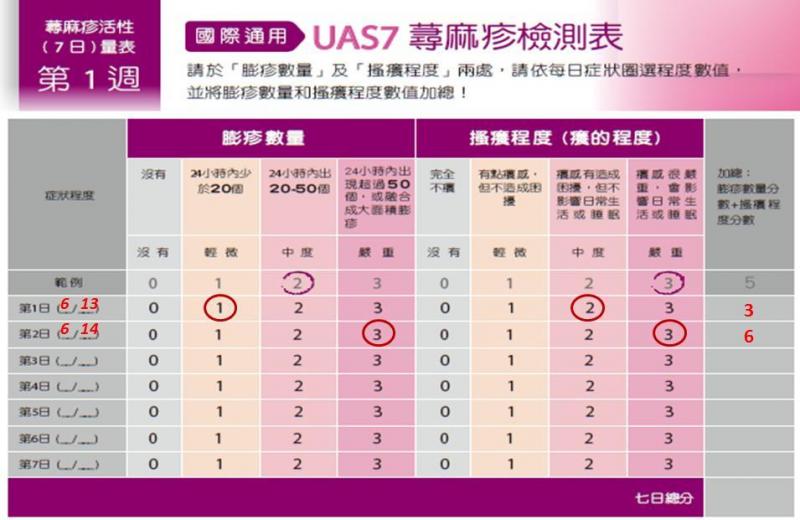
Spanish UAS7
Ideally, the Eureka Research Platform would be used for the following aspects of the online platform. Features within each section are listed in order of highest to lowest priority.
A. Electronic consent- Study enrollment
- Medical release form: On enrollment, patients will specify which providers they would like to keep informed about their hive treatment and sign a medical release form to grant these providers HIPAA-compliant access to a report of their work-up and treatment plans.
- Omalizumab (advanced CU treatment) consent form: This capability will prevent delays in care that occur when medication orders cannot be processed because patients cannot come to clinic to sign consent.
B. Symptom tracking: As there are pros and cons to each of the tools described, we will work with community partners to determine which tools will be used in the platform.
- UAS / UAS-7: Allow patients to track their symptoms daily, or even multiple times a day, a request made by users of current apps.
- Record hive triggers: Allow patients to track and identify non-allergic and physical triggers for urticaria.
- PHQ-2 / PHQ-9: Use depression screening tools to identify patients who could benefit from timely referral to psychology or psychiatry as appropriate. Up to 50% of patients with CU are affected by depression but many of them do not seek treatment. If possible, collaboration with existing online therapy apps would be ideal.
- Quality of life reports: Incorporate CU-specific quality of life surveys (CU-Q2OL, DLQI) to assess CU wellness over time.
- Smartphone-based reminders: Patients can opt in for reminders to submit their daily scores and response to medications.
C. Store patient-generated health data
- Data storage (photos of hives, photos of medication, pre-populated symptom lists, and speech-text interfaces) that reduces the need for real-time translation would improve patient-provider engagement and facilitate shared decision-making. It would clarify what treatments non-English-speaking patients are using.
- Having a report of patient-generated health data that can be recorded by patients from diverse cultural and language backgrounds would allow patients and providers to better engage in meaningful shared decision-making.
D. Facilitate communication between patients and allergists
- It would be ideal if allergists could remotely access the patient-reported data for their patients. This would allow patients to share their symptom and quality of life scores and update their allergist about what prescription, over-the-counter, and herbal medications they are taking. The allergist would take this data and recommend medication changes if necessary. A platform that could interface with Epic would streamline provider workflow, but it would be understandable if that feature is not a possibility.
- If allergists can’t access the patient-reported data unless the patient comes to clinic, the platform would still be helpful in terms of decreasing cultural and language barriers between the patient and the provider, because the app would store patient-generated health data (symptom-tracking data, medication use data, and possible triggers for the hives) that could be reviewed during the appointment. However, in this case the app would not help in terms of eliminating distance and time barriers for patients.
E. Facilitate data transfer between patients, allergists, and PCP providers
- The platform would generate a report of patients' work-up and treatment plans that can be faxed to their primary care providers for the allergy office.
- The platform would also serve as an updated physical “medical file” that the patient could show any provider during in-person clinic visits. As such, especially if the platform could interface with Epic, this study would also be an exploration into whether smartphone-based health data owned and carried by patients is indeed an effective way to store health data so that it is accessible by patients and their providers.
F. Linkage to EHR
- We could connect treatment response in our cohort to clinical data, such as autoantibody levels, to better understand the biology of CU.
Partnership with community advisors will be integral to the success of our proposed engagement platform for CU patients. As one of the highlights of our proposed platform is that it will be accessible by diverse populations, it is imperative that we work with community partners to gauge their interest in our project and to ask for their input regarding the platform.
- Primary care physicians at UCSF
- Primary care physicians in the San Francisco Bay Area
- Primary care physicians who work at clinics focused on care for patients of Chinese backgrounds (Chinatown Public Health Center in SFHN, Chinese Hospital, etc.)
- Chinese traditional medicine and acupuncture clinics in San Francisco
- Local organizations such as Asian Alliance for Health, Asian Health Institute, Chinese Community Health Resource Center, and Southeast Asia Research Institute.
- Patients with chronic hives
- English-speaking
- Chinese-speaking
- Spanish-speaking, if initial pilot with Chinese-speaking population is successful
- What can we do to make this engagement platform useful for your patients and for you?
- Do you think that it will be helpful to broaden recruitment to chronic hive patients who are not referred to allergy practices?
- What outcome measures would you be interested in knowing?
We would like to ask traditional medicine and acupuncture clinics about their current practices and how they might be integrated into our platform. Patients are increasingly interested in the role of complementary and alternative medicine (CAM) in treating atopic conditions such as hives. It is also important to improve our understanding of how patients, particularly Asian patients, perceive and use CAM.
- What can we do to make this engagement platform useful for you?
- Which symptom-tracking and quality-of-life tools would you want in the platform?
- We would also discuss any specific culturally important aspects of care that patients would like integrated into an engagement platform.
- Is there a clinically relevant difference at week 8 in the Urticaria Activity Score (UAS7) compared to baseline?
Secondary research aims:
- Investigate if there are clinically relevant differences between baseline and week 8 for other measurement outcomes requested by community partners, such as quality of life
- Obtain Kaplan-Meier estimates of the distribution of time to first minimum important difference (MID) response in UAS7 (i.e. time to a reduction from baseline in ≥ 10 points) to estimate the median time to MID.
- Compare the two language cohorts to identify differences in frequency of using the platform, frequency of messaging, or effect on UAS outcome that are potentially related to language accessibility
We expect to enroll 17 patients to detect a mean change in UAS7 at 8 weeks from baseline of 11 points (standard deviation 9 points) with 90% statistical power. This is based on a paired t-test, alpha of 0.05. A total of 34 patients will be recruited, 17 in each subgroup (English-speaking and Chinese-speaking patients). We would be able to enroll this number of patients within three months given the volume of hive patients we see. The minimal clinically important difference for the UAS7 is 10-11 points, and the standard deviation of the mean UAS7 at baseline in CU studies is 9 points. If we are able to combine the two cohorts, Asian speaking and English speaking, and/or enroll 20 people in each subgroup, we will explore the trajectory over time using a linear mixed-effects regression model.
As stated, if the initial pilot study is successful and community partners show interest, we will obtain qualitative feedback from current Spanish-speaking Latin@ chronic hive patients to tailor the platform design and function to the Latin@ population and perform a feasibility pilot study with Spanish-speaking patients.
Next steps: Randomized control trial (RCT)
If the feasibility studies are successful in showing a clinically relevant difference at week 8 in the Urticaria Activity Score (UAS7) compared to baseline, our long-term goal is to conduct a randomized controlled clinical trial (RCT) with a non-intervention group. The intervention arm would consist of patients treated by allergy providers who offer the platform, and the control arm would consist of patients treated by allergy providers who do not offer the platform and track patient symptom scores using paper forms and analog patient diaries. The clinic scheduler will randomize patients referred for hives to the intervention or control arm. Prior to starting the RCT, we will work with a biostatistician to finalize the study design and statistical analyses pending community partner input and outcomes of initial feasibility studies.
Next steps: RCT Primary Aim
- Is there a clinically relevant difference at week 8 in the UAS7 between the intervention and the control group?
Next steps: RCT secondary research questions:
- Does an online platform allow patients to engage sooner with an allergist after initial referral to Allergy is placed?
- Compared to routine care (in-person visits), does an online platform for self-directed symptom tracking and clinician engagement achieve symptom control more quickly?
- Does achieving symptom control in the first 12 weeks after symptom onset result in increased likelihood of remission (complete control of symptoms without treatment) at 6 months, 1 year, 2 years, and 3 years after symptom onset?
- Are improvements in symptom control associated with improvements in the Chronic Urticaria Quality of Life Questionnaire (CU-Q2OL)?
- Saini SS, Kaplan AP. Chronic Spontaneous Urticaria: The Devil's Itch. J Allergy Clin Immunol Pract. 2018 Jul - Aug;6(4):1097-1106. doi: 10.1016/j.jaip.2018.04.013.
- Deza G, Ricketti PA, Giménez-Arnau AM, Casale TB. Emerging Biomarkers and Therapeutic Pipelines for Chronic Spontaneous Urticaria. J Allergy Clin Immunol Pract. 2018 Jul - Aug;6(4):1108-1117.
- Zuberbier T, Bernstein JA. A Comparison of the United States and International Perspective on Chronic Urticaria Guidelines. J Allergy Clin Immunol Pract. 2018 Jul - Aug;6(4):1144-1151. Epub 2018 May 18.
- Hawro T, Ohanyan T, Schoepke N, Metz M, Peveling-Oberhag A, Staubach P, Maurer M, Weller K. The Urticaria Activity Score-Validity, Reliability, and Responsiveness. J Allergy Clin Immunol Pract. 2018 Jul - Aug;6(4):1185-1190.e1. Epub 2017 Nov 8.
- Greenberger PA. Chronic Urticaria: new management options. World Allergy Organ J. 2014 Nov 5;7(1):31.
- Bernstein JA et. al. The diagnosis and management of acute and chronic urticaria: 2014 update. J Allergy Clin Immunol. 2014 May;133(5):1270-7.
- Vietri J et. al. Effect of chronic urticaria on US patients: analysis of the National Health and Wellness Survey.Ann Allergy Asthma Immunol. 2015 Oct;115(4):306-11.
- Zhong H, Song Z, Chen W, Li H, He L, Gao T, Fang H, Guo Z, Xv J, Yu B, Gao X, Xie H, Gu H, Luo D, Chen X, Lei T, Gu J, Cheng B, Duan Y, Xv A, Zhu X, Hao F. Chronic urticaria in Chinese population: a hospital-based multicenter epidemiological study. Allergy 2014; 69: 359–364.
- Liu JB, Yao MZ, Si AL, Xiong LK, Zhou H. Life quality of Chinese patients with chronic urticaria as assessed by the dermatology life quality index. J Eur Acad Dermatol Venereol. 2012 Oct;26(10):1252-7. doi: 10.1111/j.1468-3083.2011.04277.x. Epub 2011 Sep 29.
- Balañá M, Valero A, Giménez Arnau A, Ferrer M, Jauregui I, Ballesteros C; study group of EVALUAS. Validation of The Spanish Version of The Urticaria Activity Score (Uas) and Its Use Over One Week (Uas7).Value Health. 2015 Nov;18(7):A426. doi: 10.1016/j.jval.2015.09.584. Epub 2015 Oct 20. *note this was for Novartis.
- Chronic Hives by the American Academy of Dermatology. https://www.aad.org/members/aad-apps/chronic-urticaria-for-public.
- CIU Tracker. https://itunes.apple.com/us/app/ciu-tracker/id977990786?mt=8
- Target My Hives. http://myhives.com/en/
Commenting is closed.

Comments
Thanks for taking the time to
Thanks for taking the time to submit. Would it be possible for you to share a 150-word plain language summary of your idea? How would you describe this to your next door neighbor?
Our selection committee has both UCSF and non-UCSF community-based members so a short accessible summary will make it easier for everyone to engage.
CU-HIVES -- Chronic Urticaria
CU-HIVES -- Chronic Urticaria: Helping Improve Virtual Engagement Study
Imagine having hives unpredictably at work, at the gym, while trying to sleep… there is no escape. You wait 4 months to see an allergist and start medications, but it’s hard to come in for frequent follow-up, and you can’t use MyChart to contact your doctor because it’s in English. Months later, not much has changed, and you are frustrated. All too commonly, this is the experience of patients with chronic urticaria, a condition with spontaneous, itchy hives and swelling that is highly disruptive to their quality of life.
The CU-HIVES Platform will enable patients and providers to work together to address symptoms quickly, regardless of distance or language. Patients can use the online portal to track their symptoms and receive timely individualized feedback from their doctor. Importantly, the platform will be inclusive of different languages and patients with limited literacy, helping them access allergists and achieve faster and longer-lasting relief from their hives.
Have you considered
Have you considered recruiting directly from the community? What that be possible?
Thanks for your comment! Yes,
Thanks for your comment! Yes, we think that would be possible as we already receive many referrals from outside of UCSF. Once we developed a platform, this could represent the first contact with patients who are otherwise on our waitlist to be seen.
If we're understanding
If we're understanding correctly, there are barriers to having a first contact in this manner. It would be like a cold call and the person would not have an idea of what is to be expected. In the Latin@ population, especially in the immigrant community, a "warm hand-off" or in-person introduction/orientation is generally more effective.
Thanks for pointing this out.
Thanks for pointing this out. We agree that it would not be a good idea to cold-call patients for recruitment. For new patients, our plan is to initially offer the app in-person during an initial consult visit to patients who are referred to the UCSF Allergy Clinic for management of hives. Our goal is to then expand our services by offering the app to patients over the phone who are referred to the UCSF Allergy Clinic for management of hives when we call them to schedule their initial appointment. Our subsequent goal is to work with community partners (primary care physicians and patients) and offer this app as a means to accessing allergy care for hives with a UCSF Allergy Clinic provider. We will work with our community partners to determine best ways of offering the platform to our patients. Implementation of all steps (particularly the last step) will depend on community partner input.
Suggestion: chronic hives
Suggestion: chronic hives (Urticaria)?
Comment: Were the instruments culturally adapted for Chinese Americans. If not, will there be plans to?
Question: What is the follow-up plan for non-UCSF patients to ensure that the patients' providers are informed?
Thanks for raising these
Thanks for raising these points! We have tried to clarify in our proposal that CU stands for chronic hives / urticaria.
Cultural appropriateness of instruments for Chinese Americans
There are tools that have been translated into Chinese and studied in Chinese populations. There are also tools that we would need to translate and do field testing in our population to make sure they are understandable and reliable. We have outlined these tools below. We would ask for community partner input (patients, allergists, primary care physicians) to determine which tools would be preferred for use in the app. Lulu Tsao, who is fluent in English and Chinese, will conduct semi-structured interviews with Chinese and Chinese American patients to gauge their interest in an app, how they would want to use an app (symptoms, messaging, photos), and whether they would want recommendations from an allergist via an app.
The UAS is a short questionnaire that has been used in research studies in both mainland China and Taiwan. It was used in a cross-sectional survey of about 3000 patients in tertiary hospitals in different provinces [1].
The DLQI has also been used for Chinese patients. The survey is available in numerous languages and there is an official Chinese (traditional and simplified) version available online that was translated forward and backward in Taiwan and is publically accessible. This version was studied among 148 patients with CU in Taiwan and found to have high reliability [2].
There are additional urticaria symptom and quality of life scoring tools such as the Urticaria Control Test (UCT) and the CU-Q20L. The UCT is available in 30 languages and consists of only 4 questions, although the translated versions are not readily available. The CU-Q20L has not been translated or validated in Chinese populations, and translation and field testing with our population is needed to make sure it is understandable and reliable.
As there are pros and cons to each of these tools, we will work with community partners to determine which tools will be used in the app.
If our initial pilot is successful, our plan is to also expand the platform to include Spanish-speaking patients. At that point, similar review of existing symptom and quality of life scoring tools and discussions with the appropriate community partners will be undertaken to determine which tools are most suitable for this patient population. In terms of already validated tools, the UAS and UAS7 were validated by researchers in Spain in a multicenter study of 166 patients and found to have good internal consistency, reliability, and sensitivity to change [3].
Chinese UAS7
Spanish UAS7
[1] Zhong H, Song Z, Chen W, Li H, He L, Gao T, Fang H, Guo Z, Xv J, Yu B, Gao X, Xie H, Gu H, Luo D, Chen X, Lei T, Gu J, Cheng B, Duan Y, Xv A, Zhu X, Hao F. Chronic urticaria in Chinese population: a hospital-based multicenter epidemiological study. Allergy 2014; 69: 359–364.
[2] Liu JB, Yao MZ, Si AL, Xiong LK, Zhou H. Life quality of Chinese patients with chronic urticaria as assessed by the dermatology life quality index. J Eur Acad Dermatol Venereol. 2012 Oct;26(10):1252-7. doi: 10.1111/j.1468-3083.2011.04277.x. Epub 2011 Sep 29.
[3] Balañá M, Valero A, Giménez Arnau A, Ferrer M, Jauregui I, Ballesteros C; study group of EVALUAS. Validation of The Spanish Version of The Urticaria Activity Score (Uas) and Its Use Over One Week (Uas7).Value Health. 2015 Nov;18(7):A426. doi: 10.1016/j.jval.2015.09.584. Epub 2015 Oct 20.
Follow-up plan for non-UCSF patients to ensure patient’s providers are informed
As our plan is to initially offer the app to patients who are referred to the UCSF Allergy Clinic for management of hives, it will be standard of care to communicate patient care updates with the referring providers.
Furthermore, as part of the platform, we envision that patients will be able to specify which providers they would like to keep informed about their hive treatment, and sign a medical release form granting HIPAA-compliant transfer of their patient information. Ideally, the platform would be able to interface with Epic and/or generate a report of patients’ work-up and treatment plans that can be faxed to their primary care providers, but it would be understandable if that feature is not a possibility.
Finally, the platform would also serve as an updated physical “medical file” that the patient could show any provider during in-person clinic visits. As such, especially if the platform could interface with Epic, this platform is also an exploration into whether smartphone-based health data owned and carried by patients is indeed an effective way to store health data so that it is accessible by patients and all their providers.
Thanks for sharing these
Thanks for sharing these instruments, and for submitting this proposal. I see that the language in the Spanish version is not in a form of Spanish used by most Spanish-speaking immigrant populations of the SF Bay Area. I imagine partnerships with local organizations that serve those communities and work with patients and local providers who serve them could be very helpful in refining these tools. Do you anticiapte within the one year scope of this funding you could test and refine the Chinese language tools and still have time to work on Spanish language tools?
Thank you - Our goal is to
Thank you - Our goal is to complete initial feasibility pilot studies within a year (start with English and Chinese in parallel and then Spanish) so that we will have a workable platform by the following year, although this is dependent on conversations with community partners.
I like this proposal - I
I like this proposal - I think it would help us explore ways to tailor symptom-tracking so that it is culturally appropriate and effective at ongoing engagement of participants (this is a major challenge generally). If you want to support different languages, do you already have different language versions of the validated questionnaires that you want to use?
Response: Thank you for the
Response: Thank you for the positive comment! We also are hopeful that this would be a first step in helping assess the cultural appropriateness of smartphone-based health data and best strategies for patient engagement across diverse populations.
We replied above with some information about different language versions of the validated questionnaires for hives, and will copy that information below as well:
There are tools that have been translated into Chinese and studied in Chinese populations. There are also tools that we would need to translate and do field testing in our population to make sure they are understandable and reliable. We have outlined these tools below. We would ask for community partner input (patients, allergists, primary care physicians) to determine which tools would be preferred for use in the app. Lulu Tsao, who is fluent in English and Chinese, will conduct semi-structured interviews with Chinese and Chinese American patients to gauge their interest in an app, how they would want to use an app (symptoms, messaging, photos), and whether they would want recommendations from an allergist via an app.
The UAS is a short questionnaire that has been used in research studies in both mainland China and Taiwan. It was used in a cross-sectional survey of about 3000 patients in tertiary hospitals in different provinces [1].
The DLQI has also been used for Chinese patients. The survey is available in numerous languages and there is an official Chinese (traditional and simplified) version available online that was translated forward and backward in Taiwan and is publically accessible. This version was studied among 148 patients with CU in Taiwan and found to have high reliability [2].
There are additional urticaria symptom and quality of life scoring tools such as the Urticaria Control Test (UCT) and the CU-Q20L. The UCT is available in 30 languages and consists of only 4 questions, although the translated versions are not readily available. The CU-Q20L has not been translated or validated in Chinese populations, and translation and field testing with our population is needed to make sure it is understandable and reliable.
As there are pros and cons to each of these tools, we will work with community partners to determine which tools will be used in the app.
If our initial pilot is successful, our plan is to also expand the platform to include Spanish-speaking patients. At that point, similar review of existing symptom and quality of life scoring tools and discussions with the appropriate community partners will be undertaken to determine which tools are most suitable for this patient population. In terms of already validated tools, the UAS and UAS7 were validated by researchers in Spain in a multicenter study of 166 patients and found to have good internal consistency, reliability, and sensitivity to change [3].
Chinese UAS7
Spanish UAS7
[1] Zhong H, Song Z, Chen W, Li H, He L, Gao T, Fang H, Guo Z, Xv J, Yu B, Gao X, Xie H, Gu H, Luo D, Chen X, Lei T, Gu J, Cheng B, Duan Y, Xv A, Zhu X, Hao F. Chronic urticaria in Chinese population: a hospital-based multicenter epidemiological study. Allergy 2014; 69: 359–364.
[2] Liu JB, Yao MZ, Si AL, Xiong LK, Zhou H. Life quality of Chinese patients with chronic urticaria as assessed by the dermatology life quality index. J Eur Acad Dermatol Venereol. 2012 Oct;26(10):1252-7. doi: 10.1111/j.1468-3083.2011.04277.x. Epub 2011 Sep 29.
[3] Balañá M, Valero A, Giménez Arnau A, Ferrer M, Jauregui I, Ballesteros C; study group of EVALUAS. Validation of The Spanish Version of The Urticaria Activity Score (Uas) and Its Use Over One Week (Uas7).Value Health. 2015 Nov;18(7):A426. doi: 10.1016/j.jval.2015.09.584. Epub 2015 Oct 20.
Are you proposing that
Are you proposing that physicians would have access to the patient-reported data for their patients? That will be a technical challenge - possibly surmountable (we have another similar project), but if this is a requirement, it would be good to know.
Thanks for your comments! Yes
Thanks for your comments! Yes, it would be ideal if allergists could access the patient-reported data for their patients. This would allow patients to share their symptom and quality of life scores and update their allergist about what prescription, over-the-counter, and herbal medications they are taking. The allergist would take this data and recommend medication changes if necessary. A platform that could interface with Epic would streamline provider workflow, but it would be understandable if that feature is not a possibility. If allergists can’t access the patient-reported data unless the patient comes to clinic, the platform would still be helpful in terms of decreasing cultural and language barriers between the patient and the provider, because the app would store symptom-tracking data, medication use data, and possible triggers for the hives that could be reviewed during the appointment. Having a report of this patient-generated health data that can be recorded by patients from diverse cultural and language backgrounds would allow patients and providers to better engage in meaningful shared decision-making.
One thing to point out - this
One thing to point out - this proposal (unlike the others) plans to use Eureka as an INTERVENTION, I think. The tracking, etc might actually lead to improvements in care and symptoms over time, and you'll test this. Two questions: 1) Will you be randomizing patients to a control arm? and 2) Besides the question I asked above about access to data by treating physicians, are there other aspects of the intervention that you are planning on Eureka delivering?
Our goal is to perform a
Our goal is to perform a randomized clinical trial with a non-intervention group after qualitative field testing and then initial feasibility pilot studies. We will first obtain qualitative feedback with semi-structured interviews from current chronic hive patients in our clinic to guide platform design and function. Then, we will perform initial feasibility pilot studies in English-speaking and Chinese-speaking patients. If the initial pilot study is successful and community partners show interest, we can obtain qualitative feedback from current Spanish-speaking Latin@ chronic hive patients to tailor the platform design and function to the Latin@ population and perform a feasibility pilot study in Spanish-speaking patients. Based on the results of the feasibility studies and community partner input, our long-term goal is to perform a randomized clinical trial with a non-intervention group.
The components to our intervention that we ideally plan on Eureka delivering include (1) symptom tracking (2) increased frequency of contact with patients (if that is technically possible using Eureka) (3) consent for advanced medications such as omalizumab to prevent delays in care that occur when patients cannot come to clinic to sign consent and medication orders cannot be processed (4) image transfer to reduce the dependence on translated materials and (5) use of a digital platform to store patient-generated health data. These components could be associated with improved outcomes even without a digital platform. However, analog symptom tracking is cumbersome for patients, and follow-up with allergists more frequently than once every 1-3 months is not possible given the current patient demand / provider supply imbalance. Thus, we believe that a digital platform is the most powerful way to deliver this intervention.
Will there be incentives for
Will there be incentives for patients to participate in this research study? Participation in a longitudinal study is a substantial time commitment and lack of incentives will lead to low recruitment and retention rates, which affect findings.
Thank you for the comments
Thank you for the comments and questions. In particular, the first comment provides us the opportunity to address one of the difficulties in our study.
We currently do not have resources to offer monetary incentives to patients. However, our hope is that the possibility of better immediate and long-term symptom control will encourage participation. We often hear from our chronic hive patients that inadequate communication with previous providers is one of the most frustrating things about their patient experience with chronic hives, and that patients want a way to keep track of their hives better along with their provider. In a study of Taiwanese CU patients, over half changed their care provider (most more than one time) because they expected better treatment efficacy, which speaks to the frustration with symptom control. Therefore, an engagement platform for chronic hive patients is likely something that patients will want to participate in.
Retention rates may be more of a problem, as patients who achieve hive remission will likely stop using the platform. Fortunately, obtaining multiple post-intervention measurements will be built into the platform. If we see patients obtaining symptom-free periods before drop-out, this will support our hypothesis that patients who dropped out stopped using the platform because they achieved hive remission. Furthermore, as we are starting this study in our own patient population, we will be seeing these patients for follow-up and can still measure the primary outcome (symptom control one year after initial referral to Allergy is placed) in both the control and intervention groups.
Approximately how many
Approximately how many patients do you want/need to recruit for findings? What is the estimate of number of San Franciscans, and if possible, among minority groups, who may have chronic urticaria?
Recruitment:For initial
Recruitment:
For initial feasibility studies, we need to recruit 17 people, and would ideally want to recruit 20 people (please see our updated proposal for power calculation performed with assistance of CTSI biostatistician). For our RCT, we will tailor the primary outcome and secondary outcome measures based on community partner input and the results of our initial pilot studies, and so we did not calculate specific sample size with a biostatistician at this time. We understand that the sample size needed for adequate power varies greatly between studies; however, as a benchmark, major clinical trials investigating the efficacy of CU medications have recruited approximately 300 patients total (for both intervention and control arms) for 95% power. We do not foresee major problems to recruitment, as there were 1684 patients seen at UCSF with the ICD-10 diagnosis code L50.1 between 7/1/2017 and 6/30/2018. This number under-represents the true number of patients seen with urticaria as many urticaria patients are labeled with ICD-10 codes for dermatitis, rash, or pruritus. We also foresee this number remaining stable or increasing in the future, as the incidence and prevalence of allergic conditions is on the rise.
There is a need for better studies investigating the incidence and prevalence of chronic urticaria in minority populations.
Prevalence:
Based on San Francisco census estimates and epidemiological data that urticaria affects 1% of the general population, approximately 8,843 people are affected with chronic urticaria in San Francisco. Asians would account for 3,000 of the 8,843 affected patients and Latin@ patients would account for 1,352.
Census estimates (as of 7/1/2017)
Population in SF (city): 884,363 --> 8,843
Asian: 33.9%
Hispanic or Latino: 15.3%
https://www.census.gov/quickfacts/fact/table/sanfranciscocitycalifornia/PST045217
Interesting proposal. We
Interesting proposal. We wondering about the following:
1) other than Mark's suggestion/question about community recruitment, how do you see the community advisors assisting you with thinking through your proposed study (engagement, recruitment, training considerations for example)?
2) Are you looking for a specific age group for this project? The use of smartphones by Latin@ elders - over 75- is definitely lower than TAY for example.. does anyone know the breakdown of smartphone usage in our diverse community? Curious to know and how that would impact who you are recruiting
3) how are you entering culturally and linguistically appropriate responses and/or app interactions? - have you field tested how the questions are interpreted? The app content will require field testing to ensure culturally and linguistically appropriate design and function - especially onhow the follow-up interface looks like.
4) confused as to why physicians would not have access to patient responses? can someone explain. Mark responded that there is a potential technical challenge - hmmmm - we don't know about the tech part to understand why doctors wouldn't have access. -- is the expectation that Drs are communicating to patient and/or other quality staff?
5) Do you plan on translating the MyChart for the study? We don't know enough of MyChart and its use however we do know that basic translation of any software interface is a neccesity and essential
Thank you for the comments
Thank you for the comments and questions! Please let us know if you’d like further clarification of our responses below.
1) other than Mark's suggestion/question about community recruitment, how do you see the community advisors assisting you with thinking through your proposed study (engagement, recruitment, training considerations for example)?
To answer your first question, we are planning to reach out to the following community partners to gauge their interest in our project and for their input regarding the platform.
We would like to ask primary care physicians:
We would like to ask traditional medicine and acupuncture clinics about their current practices and how they might be integrated into our platform.
Chronic hives may be of particular interest to Asian-American health organizations because chronic hives can be associated with chronic infections, such as H. pylori and hepatitis B and C, which are more prevalent among Asian populations. If of interest to primary care providers, we could discuss integration of outreach and education about these chronic infections in Asian populations with chronic hives.
We would like to ask patients with chronic hives:
2) Are you looking for a specific age group for this project? The use of smartphones by Latin@ elders - over 75- is definitely lower than TAY for example.. does anyone know the breakdown of smartphone usage in our diverse community? Curious to know and how that would impact who you are recruiting
Response:
Epidemiological studies have found that the peak incidence of hives is in a younger population, age 20-40, which is better-suited for digital intervention.
3) how are you entering culturally and linguistically appropriate responses and/or app interactions? - have you field tested how the questions are interpreted? The app content will require field testing to ensure culturally and linguistically appropriate design and function - especially onhow the follow-up interface looks like.
Response:
We definitely agree that field testing is necessary to ensure culturally and linguistically appropriate design and function. We will start by conceptualizing the initial platform design with existing symptom and quality-of-life measurement tools that have been translated into Chinese and Spanish and have been validated in Chinese and Latin@ populations. From there, we plan to use semi-structured interviews of chronic hive patients in our clinics (initially English-speaking and Chinese-speaking, and then Spanish-speaking) to guide subsequent design of the platform so that it is tailored to their cultural and linguistic needs, and to potentially adapt existing symptom and quality-of-life measurement tools to culturally diverse populations.
4) confused as to why physicians would not have access to patient responses? can someone explain. Mark responded that there is a potential technical challenge - hmmmm - we don't know about the tech part to understand why doctors wouldn't have access. -- is the expectation that Drs are communicating to patient and/or other quality staff?
Response:
Please see above response to Dr. Pletcher’s question, copied here: We are hoping that allergists will be able to access the patient-reported data for their patients. This would allow patients to share their symptom and quality of life scores and update their allergist about what prescription, over-the-counter, and herbal medications they are taking. The allergist would take this data and recommend medication changes if necessary. A platform that could interface with Epic would streamline provider workflow, but it would be understandable if that feature is not a possibility. If allergists can’t access the patient-reported data unless the patient comes to clinic, the platform would still be helpful in terms of decreasing cultural and language barriers between the patient and the provider, because the app would store symptom-tracking data, medication use data, and possible triggers for the hives. Having a report of this patient-generated health data that can be recorded by patients from diverse cultural and language backgrounds would allow patients and providers to better engage in meaningful shared decision-making.
5) Do you plan on translating the MyChart for the study? We don't know enough of MyChart and its use however we do know that basic translation of any software interface is a neccesity and essential
Response: We are not planning on translating MyChart. We will use existing translations of tools for chronic hives, and further discuss feasible methods in the Eureka platform for translating patient and physician messages. This might include offering some standardized responses and using images, to minimize the need for real-time translation of free text.
Hello. Thank you for those
Hello. Thank you for those thoughtful responses. It seems like you have thought through your approach engaging community stakeholders from physicians, clinics, and patients. I just wanted to clarify that there are community advisors on the steering committee that will be weighing in on projects and working to support them. In what way do you think community advisory members on this steering committee could be helpful to your project?
Thanks for your question--we
Thanks for your question--we think there are a variety of ways that community advisors would help shape the project, including: reviewing the existing translated tools with us to help determine which are culturally appropriate for the platform; strategies for patient recruitment and outreach beyond our clinic; thinking about the role of traditional medicine and patients' health beliefs in uptake of the platform; and including issues of particular health interest to our population, e.g. the link between chronic hepatitis and chronic hives in Asian-American patients. We have asked leadership from Chinatown Public Health Center for community input on our proposal. If selected, we would solicit interest in forming a formal advisory board of community members, with input from the steering committee.
Thank you all for the great
Thank you all for the great comments, questions and clarifications.
Q1: Given that CU can be a symtom of autoimmune or malignancy, and HepB, C - have you targeted the general population in any specific way other than API population?
Q2: do HIV+/AIDS also have disporpotionate rates of CU?
Q3: I did see in a quick lit review that angio-edima can also have a co-mrbidity or be a symtom of CU? could this be a targeted population also
Q4:I gather that CU affects gen pop at 25%, have you disaggregated this by race?
Q5: given that Afrocan Americans have disprportionate chronic disease in SF and also have access challenges is there a reason you would not extend this service into AA pop?
Q1: Our goal is to create a
Thanks for your questions!
Q1: Our goal is to create a platform that can be accessed by the general population, including the API population. The relationships between CU and autoimmunity, malignancy, and infectious conditions are still being elucidated. Having a common clinical platform for all patients with CU could help gather information that will better define these connections. However, existing platforms are not easily accessible by the API population. Our proposal focuses on creating a platform that is also accessible to the API population, which allows us to target the general population (instead of just English-speaking populations).
Q2: We have not seen this relationship in the literature, but there have been cases of patients with HIV/AIDS developing CU and needing treatment with immunomodulatory agents.
Q3: Yes, as many as 50% of CU patients also have angioedema. The platform will be created for all patients with CU, and therefore will target CU patients both with and without angioedema. Interestingly, the image transfer services in our proposed platform may help providers identify a non-CU category of patients who present with angioedema only. These angioedema-only patients can be mislabeled as CU with angioedema, but do not actually have any urticaria, and require different work-up and management than CU patients. For the purposes of this study we will focus on CU with/without angioedema. If we find that many angioedema-only patients are being diagnosed via the CU platform, the platform could certainly be expanded to include angioedema-only patients as well.
Q4: We apologize that we were not clear in our proposal: hives at any time point affects 25% of the population but CU, with symptoms lasting longer than 6 weeks, affects ~1-5% of the general population. More data is needed regarding how incidence and prevalence of CU may vary between different races. One study looking at a National Wellness Survey of 270 patients with a current prescription for chronic hives had the following race/ethnicity breakdown: 68% white, 12.2% black, 7.8% Hispanic, 6.7% Asian, and 5.2% other. However, this was a very small group and limited to active treatment, not just diagnosis.
Q5: Thank you for the suggestion! We agree we should ensure our services are accessible to the African American population and we should discuss with community advisors how to tailor the platform to make it more accessible to the African American population as well. We can also make sure we outreach to African-American patients represented in our clinic cohort for input on the platform function and design. If our proposal is approved, we would greatly appreciate any suggestions regarding potential community partners who would be interested in providing input into making the platform more accessible to the African American population.